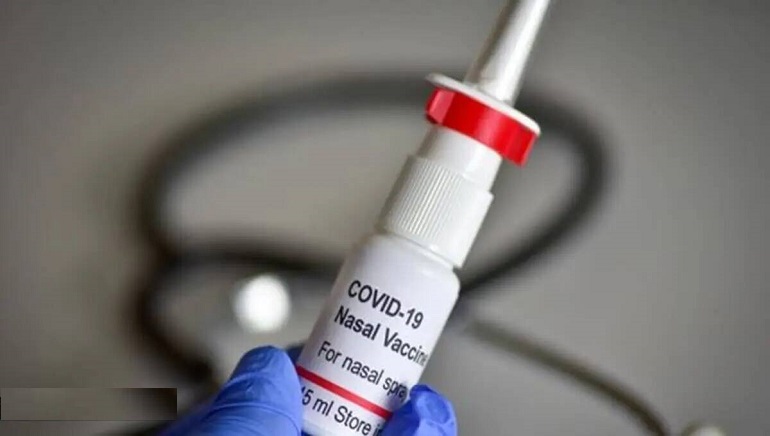
The National drug regulator has approved the country’s first intra-nasal Covid vaccine for emergency use in adults, the Government said on Tuesday.
The new vaccine, called Incovacc, is manufactured by Bharat Biotech, the company that also manufactured Covaxin. The vaccine can be administered only to the unimmunized and thus those who have already received the first and second doses of other vaccines will not be eligible to get iNCOVACC.
iNCOVACC will be administered through the nose, which might cause an immune response in the mucosal membrane. It has been designed to protect against infection and also reduce virus transmission.
The vaccine uses an altered chimpanzee adenovirus, which cannot reproduce in the body, to carry the Covid spike protein to produce immunity.
The new vaccine was developed by Bharat Biotech in partnership with Washington University-St Louis.
The vector that carries the spike protein was developed and evaluated in pre-clinical studies by the US University. Bharat Biotech is taking care of product development and manufacturing. The vaccine development was, to some extent, funded by the Department of Biotechnology’s Covid Suraksha program.
The vaccine remains stable at 2-8°C, which makes it easy to store and distribute. It will be manufactured at several places in the country, including Gujarat, Karnataka, Maharashtra, and Telangana.