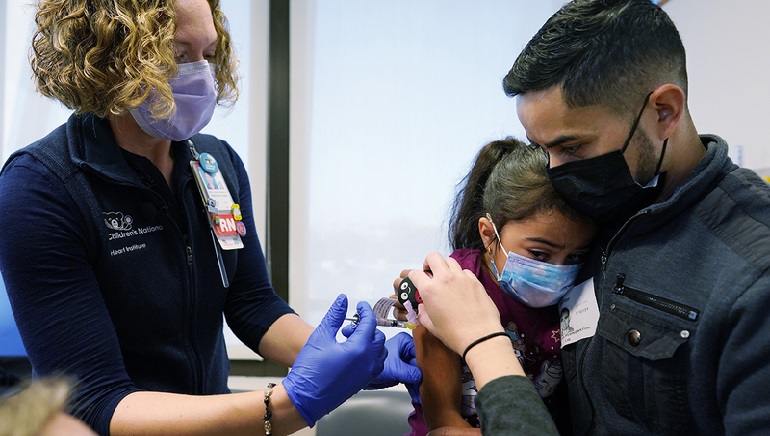
The U.S. health officials, on June 18, opened COVID-19 vaccines to infants, toddlers, and pre-schoolers- the last group to get the shots from next week.
Advisers to the Centers for Disease Control and Prevention recommended the vaccines for children as young as six months. The final nod came hours later from the Director of the agency, Dr. Rochelle Walensky, who said, “We know millions of parents and caregivers are eager to get their young children vaccinated, and with today’s decision, they can”.
Though the vaccines are approved by the Food and Drug Administration, the decision as to who gets them is determined by the CDC.
The CDC’s advisory panel said the vaccine would prevent small children from hospitalization, death, and prolonged health complications. Pfizer and Moderna are the two vaccines that got the sign-off from the FDA and the CDC. Both have different dose sizes and the number of shots.
The government is well prepared for the vaccine expansion, with millions of doses ready for distribution to physicians, hospitals, and community health clinics all over the country. Approximately 18 million kids will be eligible, but it is not known how many will ultimately get the vaccines. While Pfizer’s is for children six months to 4 years, Moderna’s is for kids six months to 5 years old.