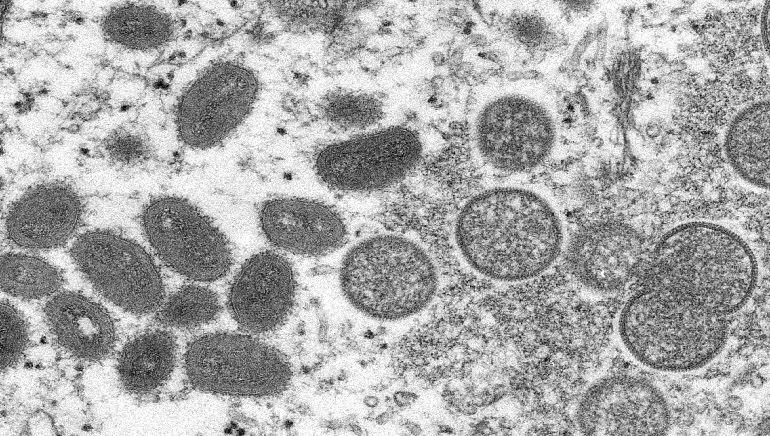
The European Medicines Agency said on Friday that the smallpox vaccine developed by Bavarian Nordic should also be used against monkeypox, as the breakout of the once-rare disease continues to affect people across the continent.
The European Union drug regulator said its suggestion was based on animal studies that testify that the vaccine protects primate animals from monkeypox. It is now the decision of the EU’s executive arm to formally approve the vaccine based on the EMA’s suggestion.
“To confirm the effectiveness of the vaccine against monkeypox, the company will collect data from an observational study that will be carried out during the ongoing monkeypox outbreak in Europe,” the EMA said. It further said that the vaccine’s safety profile was “favorable” and the pros of its use during the ongoing monkeypox outburst were far greater than the risks, with very mild-to-moderate side effects.
The vaccine, known as Imvanex in Europe and Jynneos in the United States, was already authorised for use against monkeypox by American drug regulators.
Over 15,000 monkeypox cases have been reported worldwide, out of which nearly 70% are in Europe, with over 30 countries across Europe affected.
Authorities in several countries, such as Britain, Germany, and the U.S., have provided the vaccine to health workers and those at high risk of being infected by the disease.